Questions/Graphs I need help with Data for graphs Helpful info to answer the above questions Intro I Procedure Prelab (done) From textbook Answer to prelab T1/2 = A0 / 2k T1/2 = Ln2 / k T1/2 = 1 / kA0 A 70 kg patient is treated with ciprofloxacin (2 x 500mg/day) for infection pulmonary Ciprofloxacin is an antibiotic eliminated mainly by the kidneys, its sound apparent distribution volume is 3 1/kg) and its clearance is 05 (Wh x kg) Calculate the halflife time of ofloxacin in thisDear Student, In a response at first sum is 100 % t1/2 implies the time in which it ends up noticeably half then half is remained Next time half of that half disintegrates along these lines, another t1/2 goes That is called t3/4 In this way, 2 t1/2 = t3/4 Here t1/2 = ln2/k

C Program For Half Life Determination Using Functions And File Input Stack Overflow
T1/2 ln2/lambda
T1/2 ln2/lambda- t1/2 = ln2 / k t1/2 = 0693 / t1/2 = 7372 days ardni313 ardni313 The halflife of the radioisotope 7368 days Further explanation The core reaction is a reaction that causes changes in the core structure 10%/90%=e^ k*1800 ln10%/90%/1800 = k = 395E4 t1/2= ln(2)/k t1/2 = 693/395E4= 1754s 1754s*1min/60s = 29 min
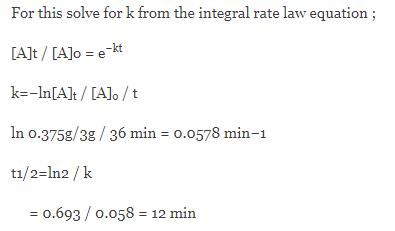



If 6 0 G Of Substance S Decomposes For 36 Minutes The Mass Of Unreacted S Remaining Is Found To Be 0 750g What Is The Half Life Of Thisreaction If It Follows First Order Kinetics
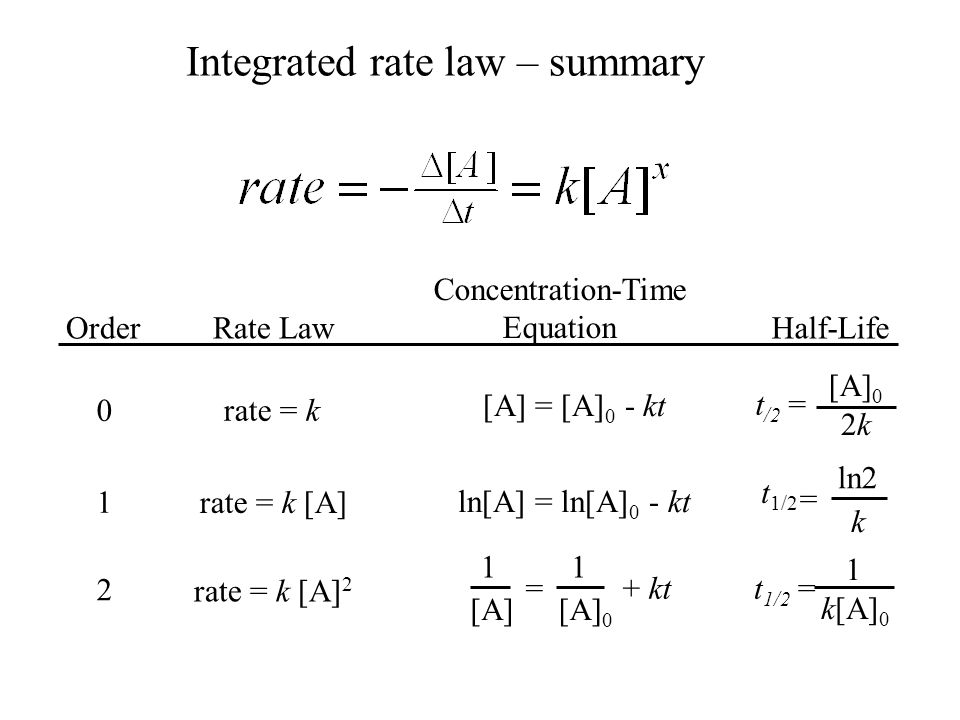
1/2=ek ·t1/2 ln2=k ·t1/2 t1 /2 =ln2/k Halflife does not depend on concentration D\NEWDDRIVE\pha5127_Dose_Opt_I\Homeworks\Homework1\Fall01\anshmwk1doc 4 Time (hr) Drug Graph indicates zero order elimination because drug concentration vs time results in a straight line Zeroorder elimination CC 0 =k ·tLn 2 is called the halflife of an exponential decay, where ?=RC is the time constant in an RC circuit The current in a discharging RC circuit drops by half whenever t increases by t 1/2 For a circuit with R=3 k?Bisection Method (21) 1 Intermediate Value Theorem If f is continuous on a, b and K is a number between f a and f b , then there exists a number c in a, b for which f c K Note that i If f a f b 0, then either f a or f b is less than 0( K), and there exists a number c in a, b for which f c 0 c is a solution of the equation f x 0 ii The condition in i is a sufficient condition, that
Once you have that, you can determine the half life, which is t1/2 = ln2/k multiply that by 3 to get the total time elapsed for 3 half lives After that, use the first order rate law lnA0 lnAt = kt and solve for At Log in or register to post comments Where, k is the rate constant of the reaction t1/2 is the halflife time of the reaction (t1/2 = 16 years) ∴ k = ln2 / (t1/2) = 0693 / (16 years) = 428 x 10⁻⁴ year⁻¹ For firstorder reaction kt = lna / (ax) where, k is the rate constant of the reaction (k = 428 x 10⁻⁴ year⁻¹) t is the time of the reaction (t = t1/2The halflife and the degradation rate constant are related by the equation t1/2 = ln2/k
The rate constant, k, will be equal to k = ln 2 t 1 / 2 so k = d a y − 1 For the reaction to be 50% complete, that will be exactly the halflife of the reaction at 10 days For the reaction to be 60% complete, using the similar equation derived from question 4, we have A t A o = 04 t = 132 d a y sT1/2 = ln2/k or k = 0693/t 1/2 applies where t 1/2 is the halflife and k is the firstorder rate constant, so k = 0693/36 hrs = hr^1 For a firstorder process rate = kA^1 where k is the firstorder rate constant and A is the amount of radioactive material present5g25g125g063g 87/29 =3 8 A 0456mg sample of hydrogen3 was collected




Chemical Kinetics Principle Of Chemistry Ii Lecture Notes Ch 302 Docsity



Rates Of Enzyme Reactions
1/2 −ln 1 2 =kt /2 ln2=kt 1/2 t 1/2 = ln2 k Note t1/2 independent of A o Means that in each interval of duration t1/2 the concentration decreases to half of what it was at the beginning of the interval, throughout the entire course of the reaction Graphical picture of half life 7 The time constant τ Defined simply as τ =1/k t/t 1/2 1The quantity t 1/2 =? Halflife for pseudo 1st order expts Expt 1 (t1/2) A = mol dm–3 Rate = (kB1) A1 Rate = k'A1 B = 0100 mol dm–3 where k' = kB1 pseudo first order ln2 t1/2 = k' ln2 = = kB ln2 k0100



2




C Program For Half Life Determination Using Functions And File Input Stack Overflow
k = ln(Af/Ai) / t, and thus T1/2 = ln2/k ln(057) / 74 min = /min, and T1/2 = ln2/0076, = 912 minutes You can mentally check this by looking at the original data You have slightly less thanT1/2 = ln2/k = ln2/0210 = 330 sec b) How long does it take to hydrolyze 75% of the sucrose?The equation can easily be remembered from the definition if said in the right way Clearance is the volume of plasma completely cleared of drug per unit time So Cl = V D x k el And rearranging Cl = V D x ln2 / t 1/2 or t 1/2 = V D




Pdf Cluster Radioactive Decay Within The Preformed Cluster Model Using Relativistic Mean Field Theory Densities



Chemical Kinetics Kinetics How Fast Does A Reaction Proceed Ppt Download
View Test Prep practice exam 2 from CHEM 102 at Drexel University Practice Exam II CHEM 102 Potentially useful data First order halflife t1/2 = ln2 / k At = kt Ao lnAt = ktThe halflife and the degradation rate constant are related by the equation t1/2 = ln2/k t1/2 = ln2/k = 0693/k So for the avidinbiotin complex t1/2= 0693/ = approx 80 days If we compare data from a range of muscarinic M3 antagonist in the table below it is clear that the reason Tiotropium displays long lasting pharmacological effects despite very low trough levels in the clinic is due to the offrate from the M3 receptor



The Nucleus Ppt Video Online Download



Half Life Wikipedia
And C=3 ?F, if the current is 6 mA at t=5 ms, at what time (in ms) will the current be 3 mA? Homework Statement Show using your expression for \\lambda that if at a time t1 the amount is y1, then at a time t1 ##\\lambda## the amount will be ##\\frac{y1}{2}##, no matter what t1 is Homework Equations y = y0 ##e^{kt}## From previous question half life ##\\lambda =ln2/k## The AttemptThus, they are only order of magnitude comparable F = female, K cond = conditional stability constant, k obs
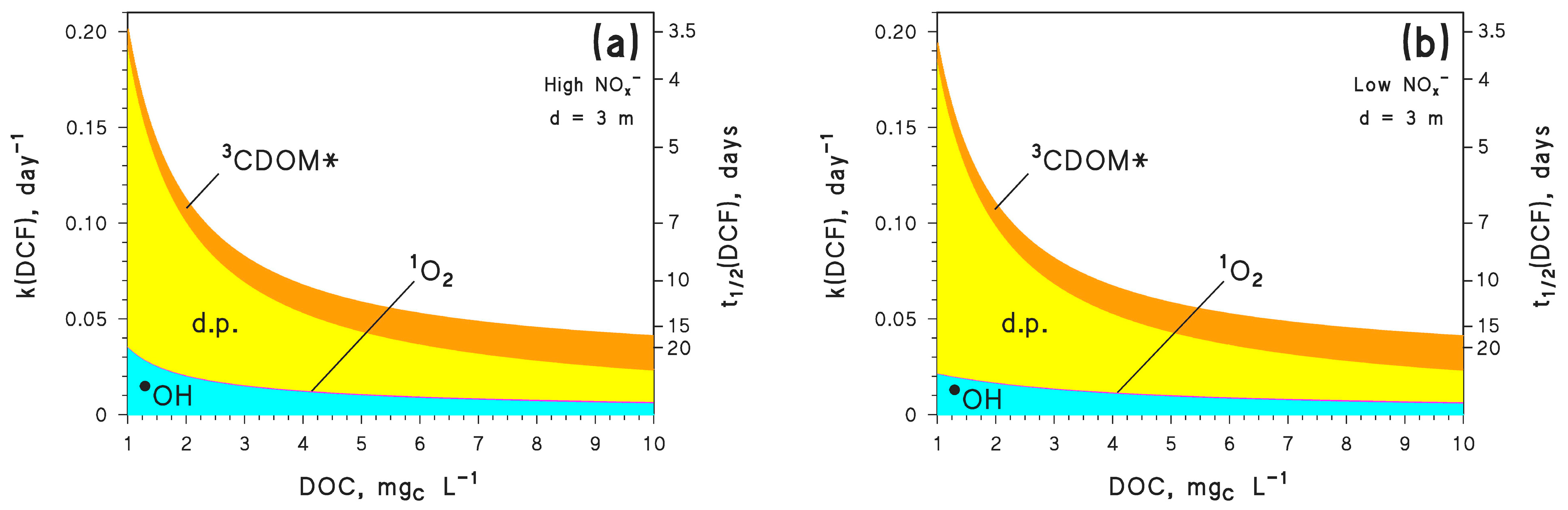



Molecules Free Full Text Insights Into The Time Evolution Of Slowly Photodegrading Contaminants Html
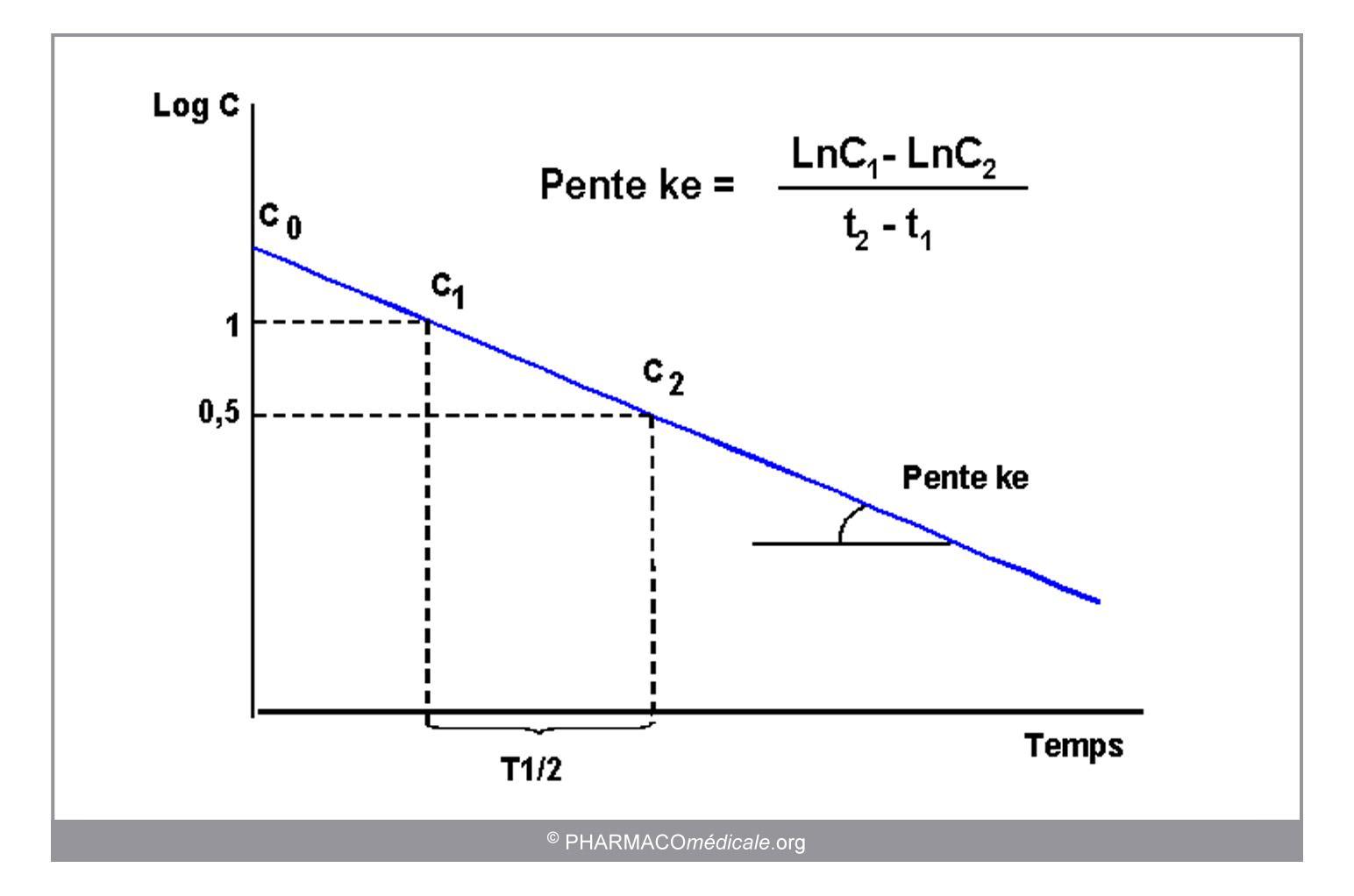



Demi Vie
T1/2 = ln2/k second order half life t1/2 = 1/(kxAo) shelf life length of time the product may safely be stored on the dispensary shelf before significant decomposition occurs, time taken for 10% active drug decomposition zero order shelf life t09 =1/2=ek ·t1/2 ln2=k ·t1/2 t1 /2 =ln2/k Halflife does not depend on concentration The equations did not have to be deduced I just did so to show from where they actually come from Title anshmwk1PDF Author Pat Khan Created Date Wednesday, AM Explanation I don't really understand the equation you mention in your question, for that I would need more information However, it might help you to know that 0693 is the same as ln(2) This is used for calculating half life / the exponential decay constant λ = ln2 T in which T is the half life of an element



Web Mst Edu



A Systematic Study Of Mocvd Reactor Conditions And Ga Memory Effect On Properties Of Thick Inal Ga N Layers A Complete Depth Resolved Investigation Crystengcomm Rsc Publishing Doi 10 1039 C9cec
Ln=ktln t1/t2=ln2/k k=ln/t 2 years It undergoes β− decay into yttrium90, with a decay energy of 0546 MeV b How much of a 50g sample of strontium90 will remain after 87 years?The radioactive isotope of lead, Pb9, decays at a rate proportional to the amount present at time t and has a halflife of 33 hours If 1 gram of thisT1/2 = ln2 / K H = 0693 / K H (5) pH Dependence From the equation (5) it is evident how pH affects the overall rate constant for hydrolysis, K H at high or low pH (high OH or H) either base or acid catalysed term is usually dominant, while at pH 7 the neutral processes can often be the most important
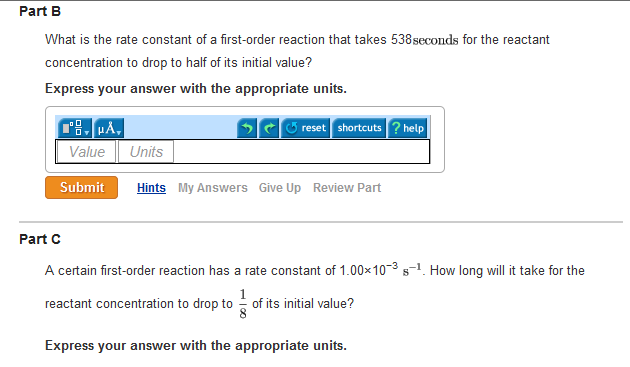



Solved Half Life Equation For First Order Reactions T1 2 Chegg Com




Nonlinear Relationship Between K And Ml Kg 1 Min 1 With A Slow Download Scientific Diagram
But Κel is also equivalent to ln2 divided by elimination rate halflife t1/2 (Κel=ln2t1/2) Thus, Cltot = ln2 Vd/t1/2 This means, for example, that an increase in total clearance results in a decrease in elimination rate halflife, provided distribution volume is constant75% = 2 half lives = 660 sec 2 In a first order decomposition reaction, 500% of a compounds decomposes in 105 min a) What is the rate constant of the reaction?How many halflives did the sample go through at the end of 87 years?



2



2
Vì phản ứng bậc nhất nên ta có kt1/2 = ln2 = 0,693 ⇒ k = k 27o C = 0, 693 = 1,3910−4 giây1 5000 b Ở 37oC, thay số ta có k 37o C = 0, 693 1 = 6, 9310−4 giây 1000Start studying chem 2 test 1 Learn vocabulary, terms, and more with flashcards, games, and other study toolsK increases as temperature increase in endothermic reactions Pressure effect on Equilibrium increase in pressure/ decrease in volume favors side of reaction with least moles




Oneclass Part A Half Life Equation For First Order Reactions T1 2 0 693k Where T1 2 Is The Half Lif



1
Rationale In the onecompartment model following iv administration the mean residence time (MRT) of a drug is always greater than its halflife (t(1/2)) However, following iv administration, drug plasma concentration versus time (t) is best described by a twocompartment model or a two exponential equationC=Ae(alpha t)Be(beta t), where A and B are concentration unitT1/2 is the halflife time of the reaction (t1/2 = 106 minutes) ∴ k = ln2/(t1/2) = 0693/106 = 654 x 10⁻² min⁻¹ For firstorder reaction kt = lna/(ax) t_"1/2"^((2)) = "8525 s" Notice how you're given the halflife (for one temperature), a second temperature, and the activation energy The key to doing this problem is recognizing that the halflife for a firstorder reaction is related to its rate constant the rate constant changes at different temperatures Go here for a derivation of the halflife of a firstorder reaction



4 What Fuels Our Cells Functions Of Cells And Human Body




Problem Set 12 Solutions Physical Chemistry I Che 360 00 Docsity
Ln2 / k first order elimination, k= slope x 2303 first order elimination ln C = ln C0 kt Dose= increases t1/2 by decreasing metabolism cardiac failure decreases clearance so increases t1/2 hepatic failure decreases elimination so increases t1/2 renal failureYou know that in every dynamic 1st order reaction (a reaction with a fixed rate of promotion) we can say that half life is when N/N0=1/2 t1/2= (ln (1/2))/k = ln2/k t1/2=0693/k This formula1 k = ln2/t 1/2 =301/14 = 2 ln A = kt lnAo = 0027*300 ln(22) = 081 3091 lA 228 v = kA lnA = 228 A = 979 µM = 0027*979 = 0026 µM



Arxiv Org
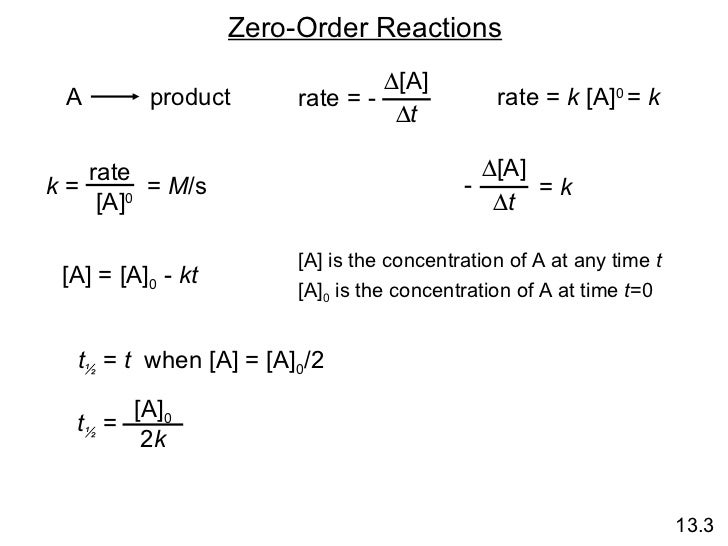



Chemical Kinetics Online
CL/Vss = K The use of K derived from the ratio of Vss and CL for halflife calculation (ln2/K) can be applied only to onecompartment model >>This is true that K is the elimination rate constant for a 1 compartment model In the case of the 2 compartment model or higher, CL/Vss = kss, the steadystate rate constant kss = 0693/t1/2ss = 0693O T = time • Can solve this equation for a specific time point – the half life At this time point t = t1/2 and N = N0/2 • Substituting into original equation gives o N0/2 = N0ekt1/2 > 2 = ekt1/2 > ln2 = kt1/2 o Therefore t1/2 = ln2/k • This explains why a large halflife corresponds to a small decay constant (and activity) 4 Calculate ages of artefacts from activity ratiosActually, you don't need to know about radioactive decay constants, λ , "k", etc to do halflife calculations However, if you must learn about these in school, then this is the place to learn it Radioactive Decay Constant λ (lambda) If you need to know about the "k" radioactive decay constant, click here, otherwise just stay here λ (lambda) is defined as the natural log of 2



2



Activation Energy
The halflife is t1/2 = ln2/k The goodness of fit of the decay model for each gene was assessed with the F statistic (ref ), based on the null hypothesis that the data fit a firstorder decay model A bootstrap method was used to calculate confidence intervals for lnC 0 – ln2 = lnC 0 –kt 1/2 ln2 = kt 1/2 t 1/2 = ln2/k where ln2 = 0693 Now what about clearance? Relevant Equations 𝑇1/2 = (𝑙𝑛2) 𝑥 𝑉𝑜𝑙𝑢𝑚𝑒 𝑑𝑒 𝑑𝑖𝑠𝑡𝑟𝑖𝑏𝑢𝑡𝑖𝑜𝑛/ clarence A 70 kg patient is treated with ciprofloxacin (2 x 500 mg / d) for an infection pulmonary Ciprofloxacin is




For A Zero Order Reaction A Rarr B T 1 2 Is K Is Rate Constant




Half Life Of A Second Order Reaction Video Khan Academy
First order (Radioactive decay) t1/2 = ln2 / k or Cheggcom Science Chemistry Chemistry questions and answers First order (Radioactive decay) t1/2 = ln2 / k or 0693 / k 1 It takes 293 hours for a radionuclide to reach 70% of its original activityNote—Halflife (T1/2) is calculated as ln2/k obs Note that K therm, K cond, k obs and T1/2 data were not obtained in identical testing, are not an exhaustive listing, and can be highly dependent on conditions;For half life, t1/2 05 = For time t99% to go to 99% completion 001 = t99% = k , 1/2 = t99% In 4605 ln2 = 0693 t1/2 ln2 k 664 For ok (Table 91) a b In a b c = 77 x 1011 X 1011 X 101 13 x 1011 x 104 = —In (fraction remaining) = kt In 2 tin = k In 2 — = yearsI t1/2 = x 25 = = 0540




For A Zero Order Reaction A Rarr B T 1 2 Is K Is Rate Constant




Cellular Cholesterol Efflux Mediated By Cyclodextrins Demonstration Of Kinetic Pools And Mechanism Of Efflux Sciencedirect



Solved How Can I Calculate The Half Life Of The Crystal Violet With Hydroxide Reaction Course Hero
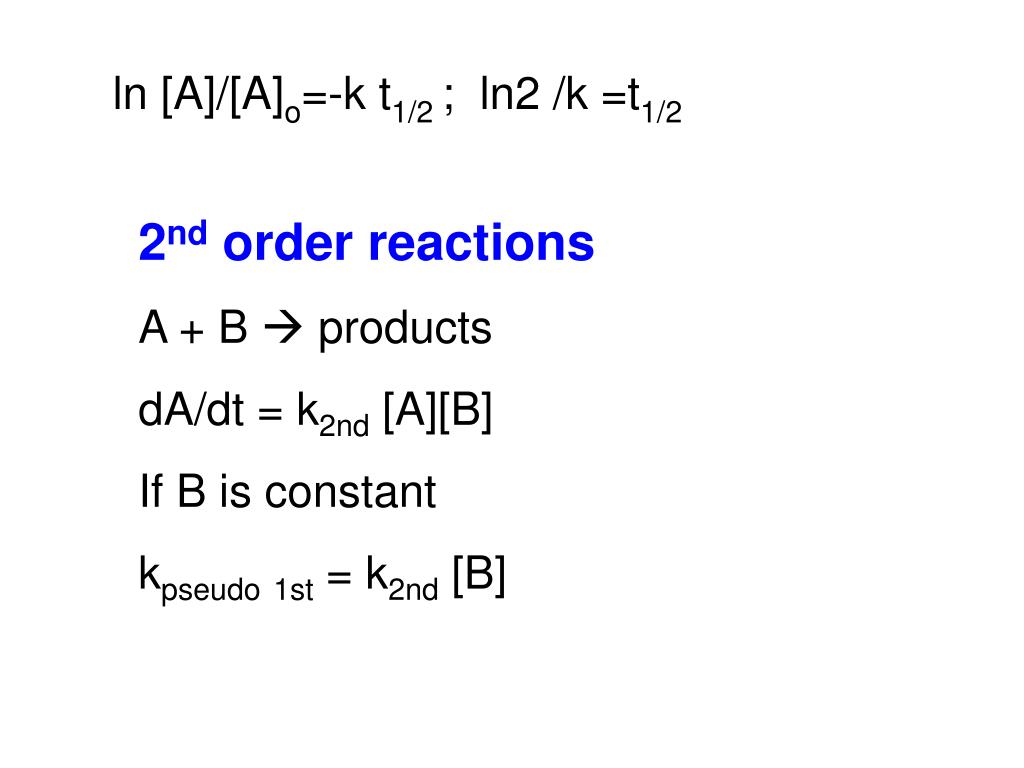



Ppt Class Objectives Powerpoint Presentation Free Download Id
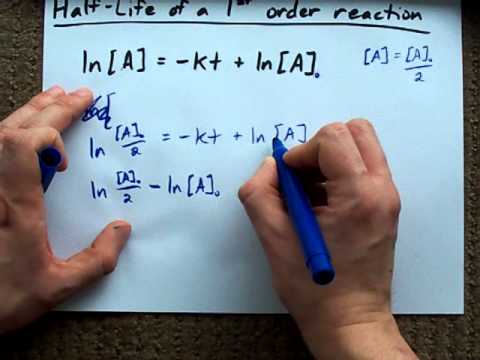



Half Life Of A First Order Reaction Derivation Youtube
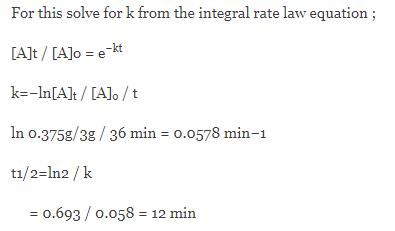



If 6 0 G Of Substance S Decomposes For 36 Minutes The Mass Of Unreacted S Remaining Is Found To Be 0 750g What Is The Half Life Of Thisreaction If It Follows First Order Kinetics
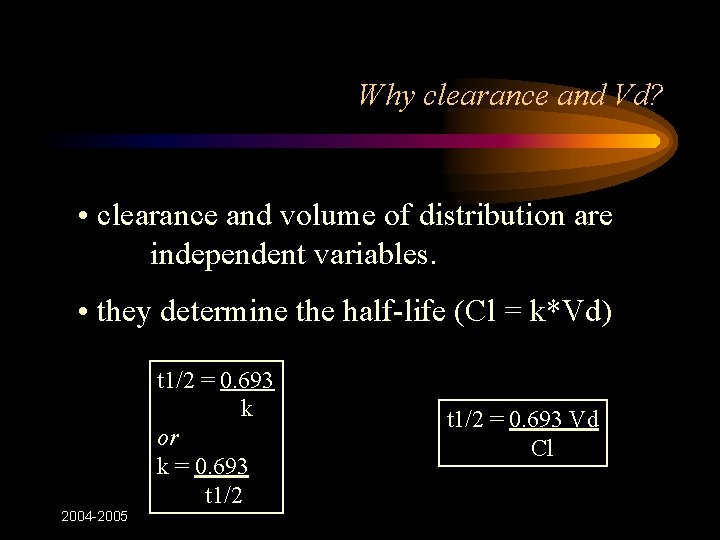



Module 2 3 Pharmacokinetics 04 05 How To




In A First Order Reaction A Rarr B If K Is The Rate Constant And Initial Concentration Of The Reactant Is 0 5 M Then Half Life Is
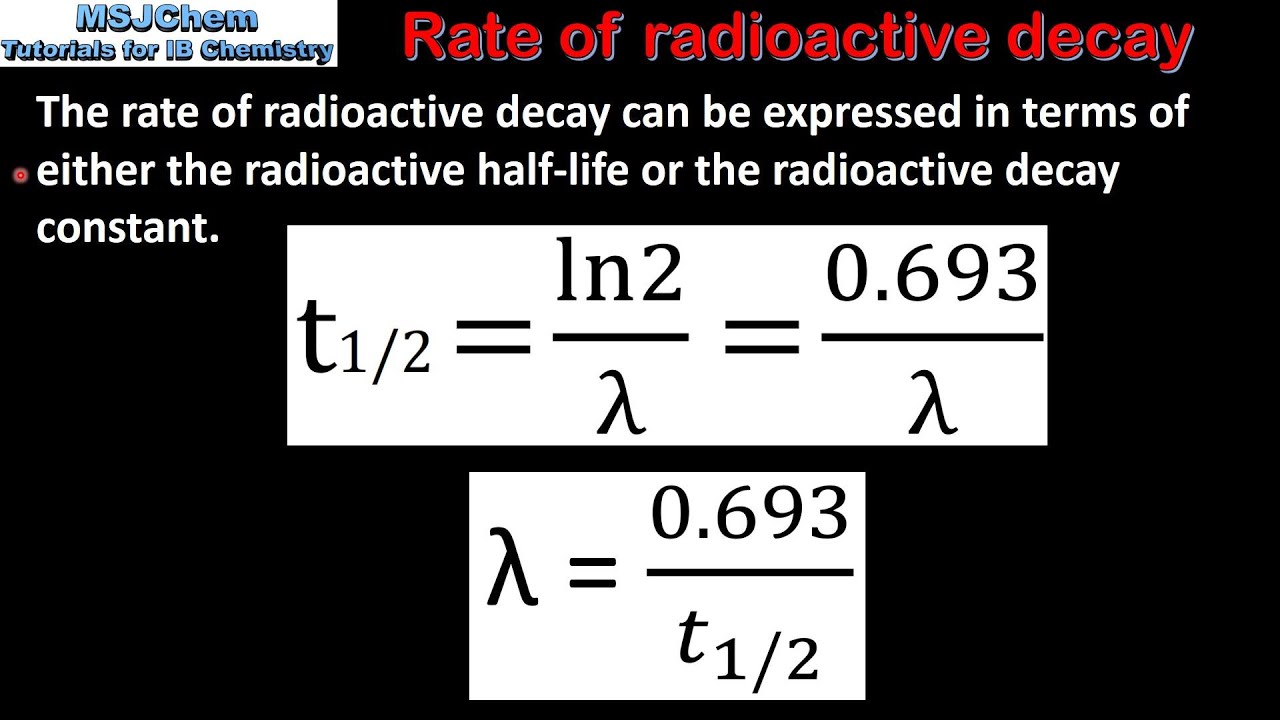



C 7 Rate Of Radioactive Decay Hl Youtube




Which Order Reaction The Half Life T1 2 Is Independent Of The Initial Conc Of The Reactants
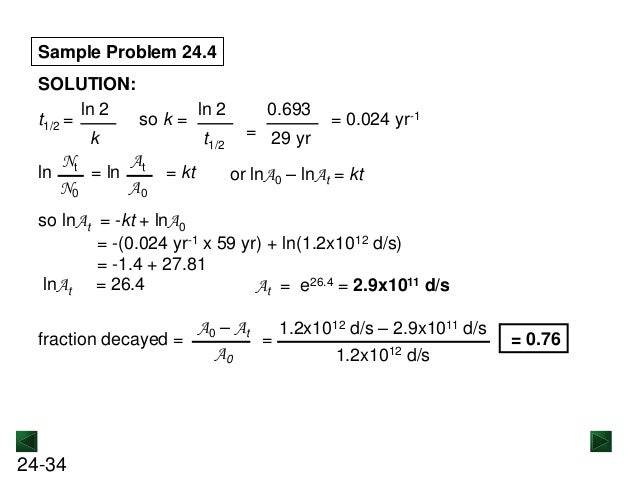



New Chm 152 Unit 10 Nuclear Chemistry Power Points Su13




Solved T1 2 Ln2 K Show All Work Calculate Rate Chegg Com




Ppt Chemical Kinetics Ch 13 Powerpoint Presentation Free Download Id




Nuclear Fusion And Radiation Pdf Free Download




Half Life Of A First Order Reaction Video Khan Academy



9 E Chemical Kinetics Exercises Chemistry Libretexts
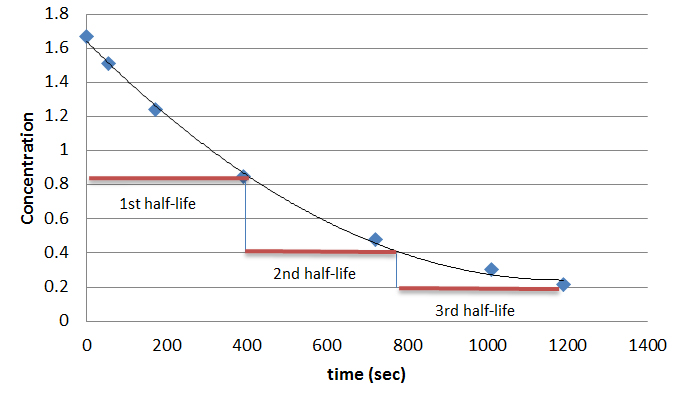



The Half Life Of A First Order Reaction Is 1 5 Hours How Much Time Is Needed For 94 Of The Reactant To Change To Product Socratic
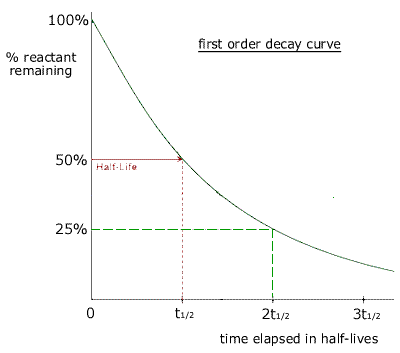



Kinetics 6 34 Half Life



Half Life Equations
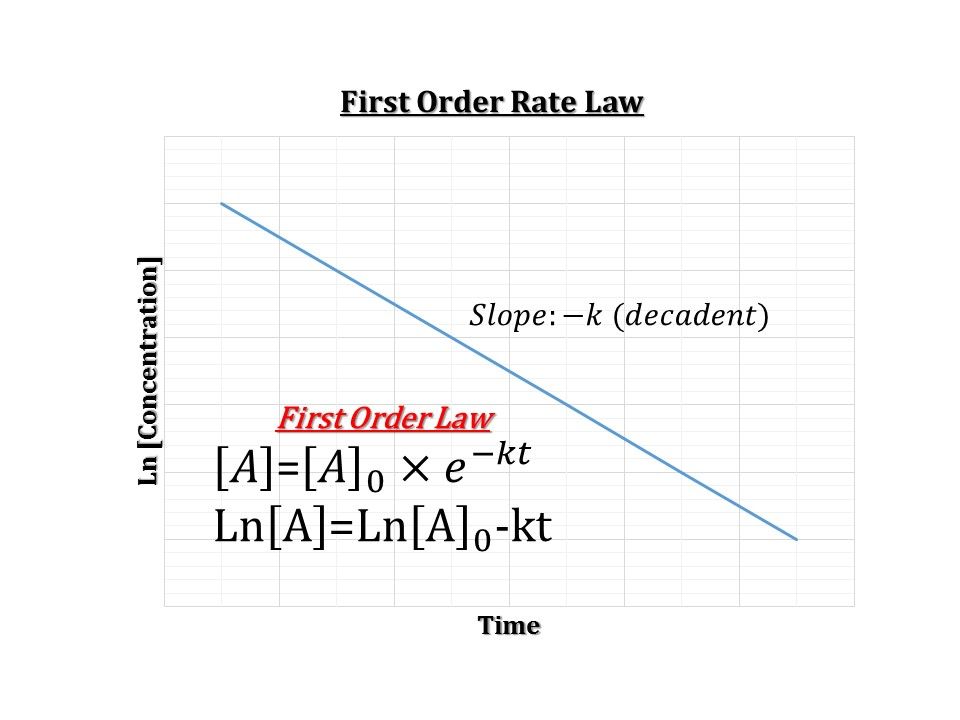



Help With Chem 2 Lab Please Thanks Half Life Chegg Com



Half Life Equations
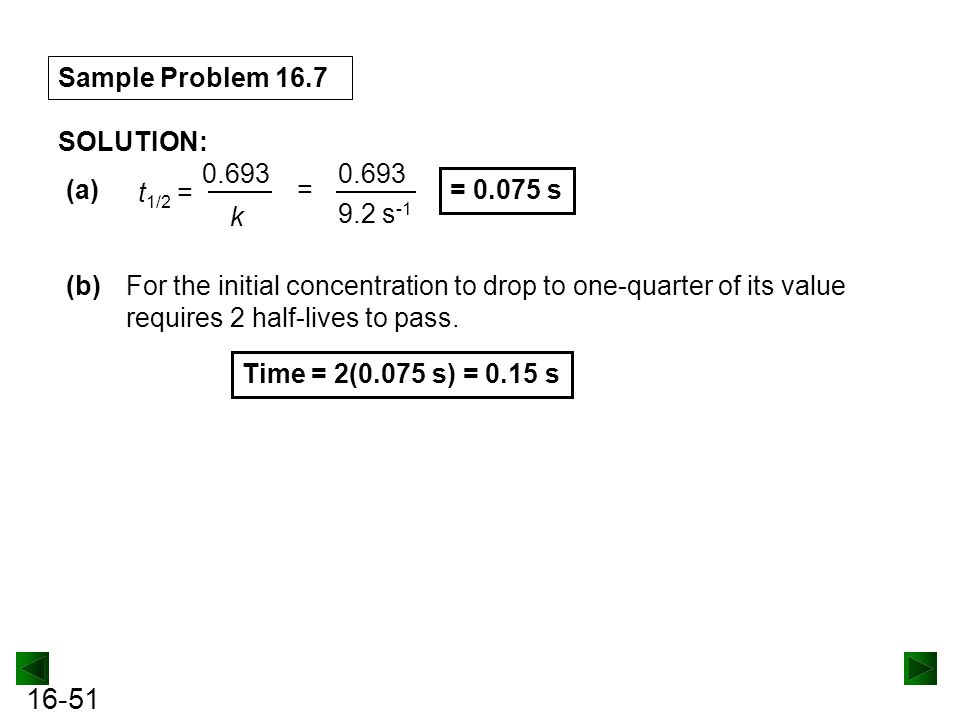



Chapter 16 Kinetics Rates And Mechanisms Of Chemical Reactions Ppt Download




Chemical Kinetics Chapter Ppt Video Online Download
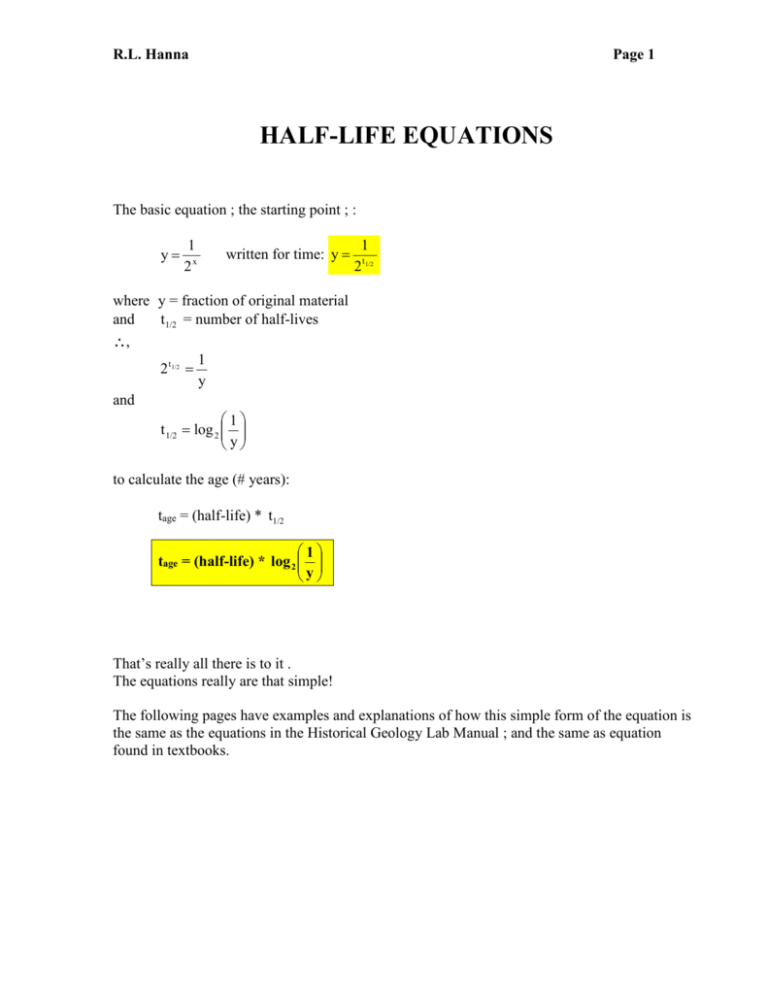



Half Life Equations



What Is The Effect Of Reactant Concentration On The Half Life Of A First Order Reaction Quora
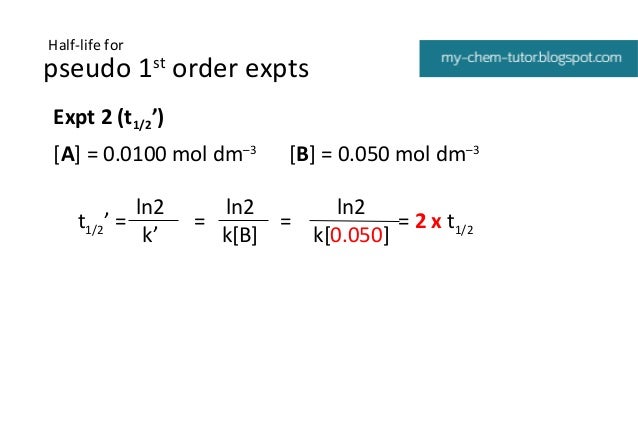



Kinetics Pseudo Order
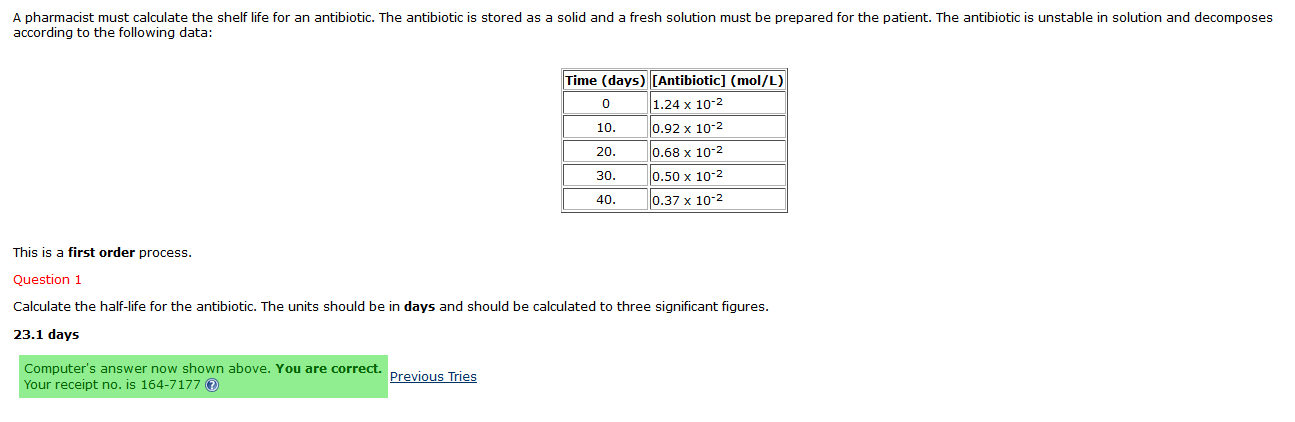



Solved 1st Order Half Life Problem Integrated Rate Law Chegg Com




Boiling Point Correction For Ln 2 Different Radiometer Manufacturers Download Scientific Diagram




Elimination Rate Constant An Overview Sciencedirect Topics




Measurement Of Rate Constants Ppt Download




Half Life Wikipedia




Lecture Slides On Chemical Kinetics General Chemistry Chem 162 Docsity




Elimination Rate Constant An Overview Sciencedirect Topics




Chemical Kinetics Chapter 13 Copyright C The Mcgraw Hill Companies Inc Permission Required For Reproduction Or Display Ppt Download
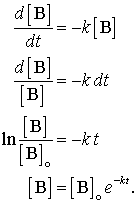



Activation Energy




Dissociation Half Lives T Ln2 K D Of Cu Dotpi And Download Scientific Diagram
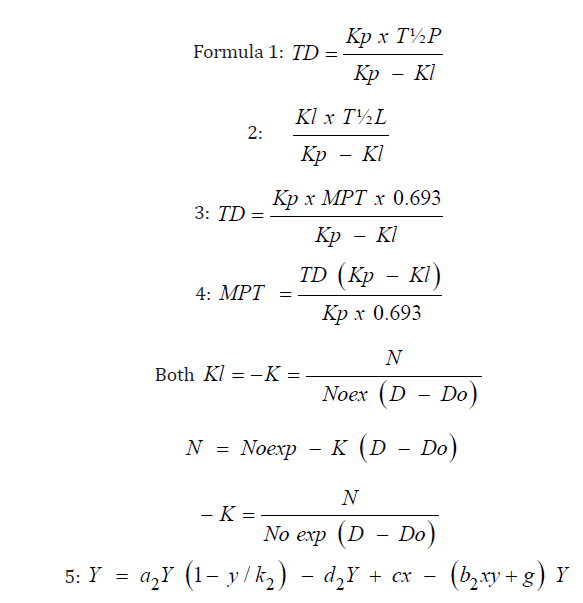



A Review Of Mathematical Concepts For Calculation Of Cancer Parameters



Math Half Life Formula



Jimmunol Org



What Is Half Life Of Radioactive Element Prove That T1 2 0 693 Lambda Where Lambda Is Disintegration Constant Quora




Chemical Kinetics Chapter 13 Dr Ali Bumajdad Chapter 13 Topics Rate Of A Reaction Reaction Rates And Stoichiometry The Rate Law Relationship Between Ppt Download
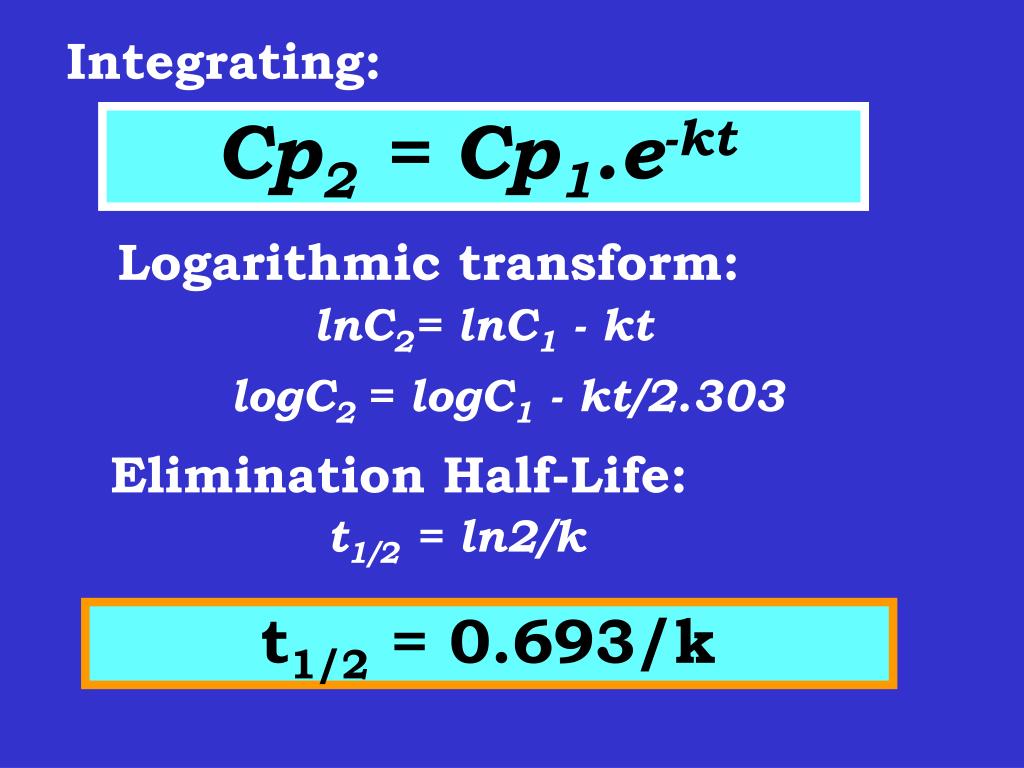



Ppt Clinical Pharmacokinetics Powerpoint Presentation Free Download Id



2



Half Life Equations
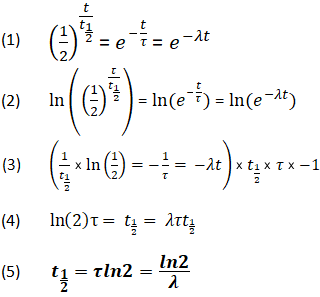



Half Life Calculator



3



Sympnp Org
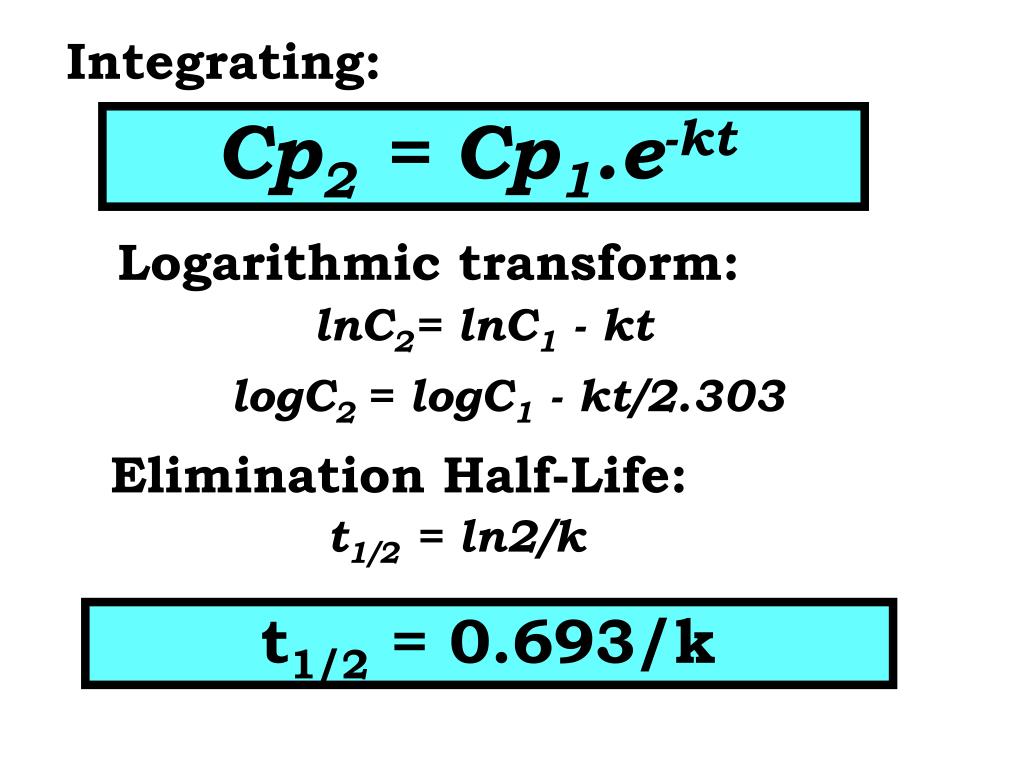



Ppt Pharmacokinetics Powerpoint Presentation Free Download Id 2215




Chapter 4
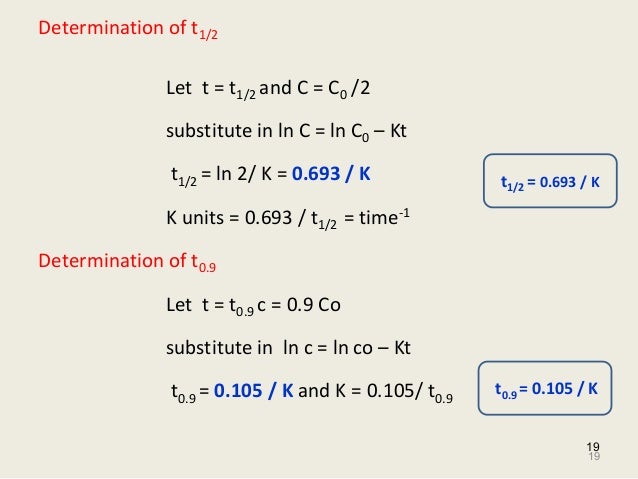



Kinetics And Drug Stability Ed



What Is Half Life Of Radioactive Element Prove That T1 2 0 693 Lambda Where Lambda Is Disintegration Constant Quora




Half Life Wikipedia
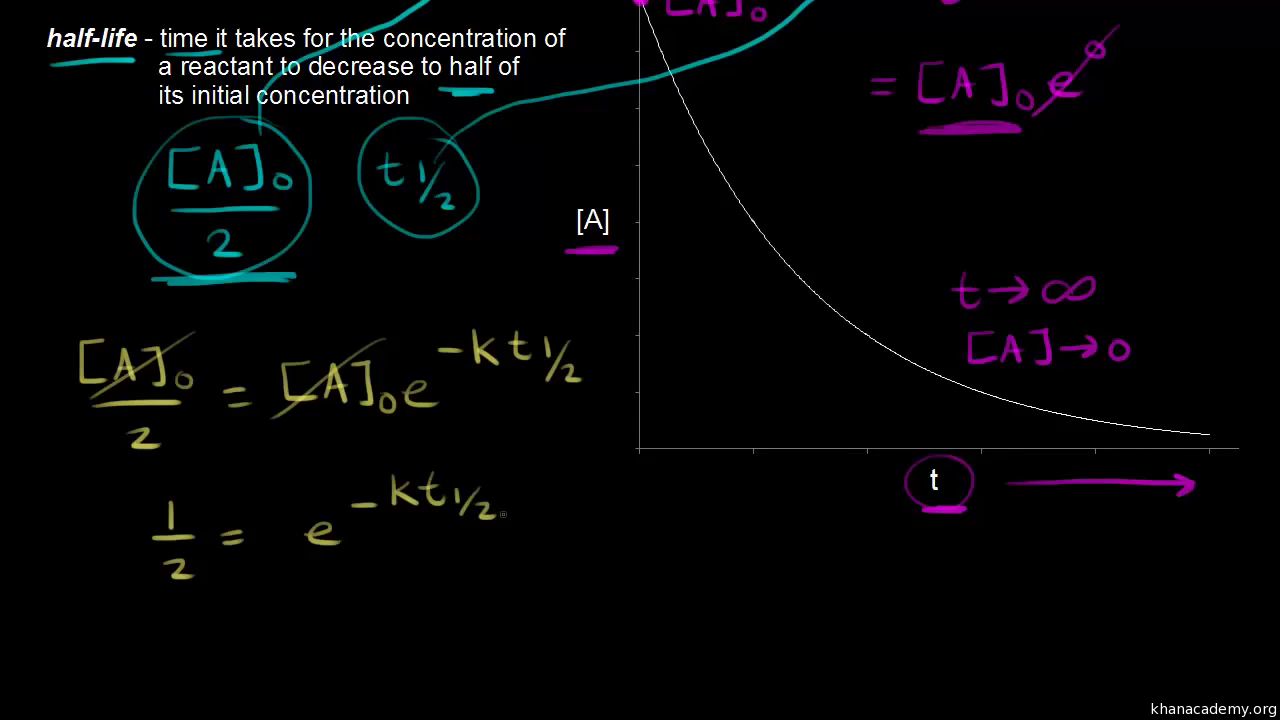



Half Life Of A First Order Reaction Video Khan Academy
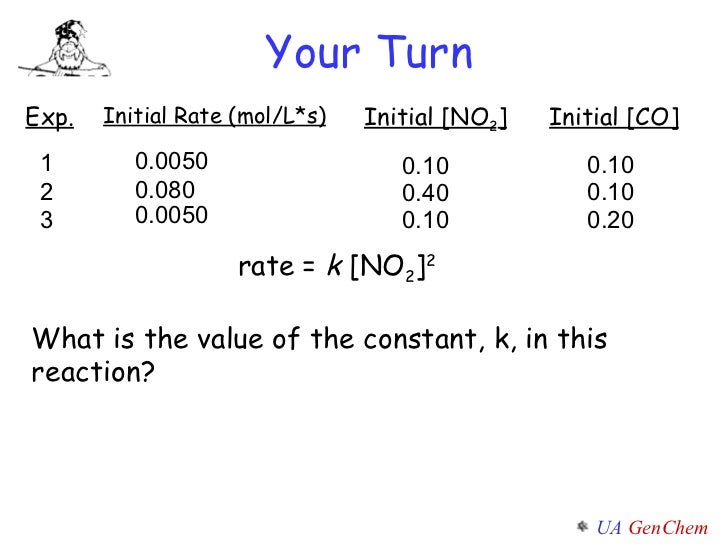



Lect W2 152 Rate Laws Alg




Hnrnp L Dependent Protection Of Normal Mrnas From Nmd Subverts Quality Control In B Cell Lymphoma The Embo Journal
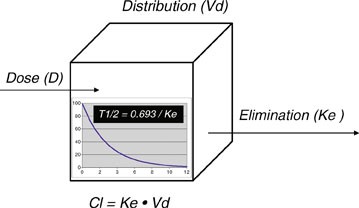



Pharmacokinetics Springerlink




The Decomposition Of N2o5 Dissolved In Carbontetrachloride Occurs As Follows N2o5 Solution 2no2 Solution 12o2 G This Reaction Is Of First Order And Its Rate Constant Is 5 0 10 4 Sec 1 If Initial




Week 12 Pharmacokinetics Ii Quantitative Clinical Pharmacokinetics Flashcards Quizlet




Kinetics Flashcards Quizlet



2



2
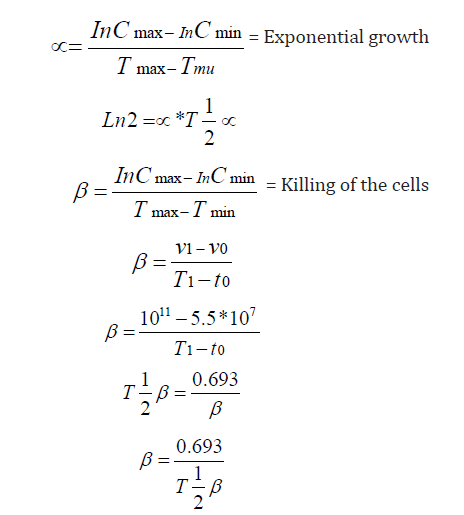



A Review Of Mathematical Concepts For Calculation Of Cancer Parameters
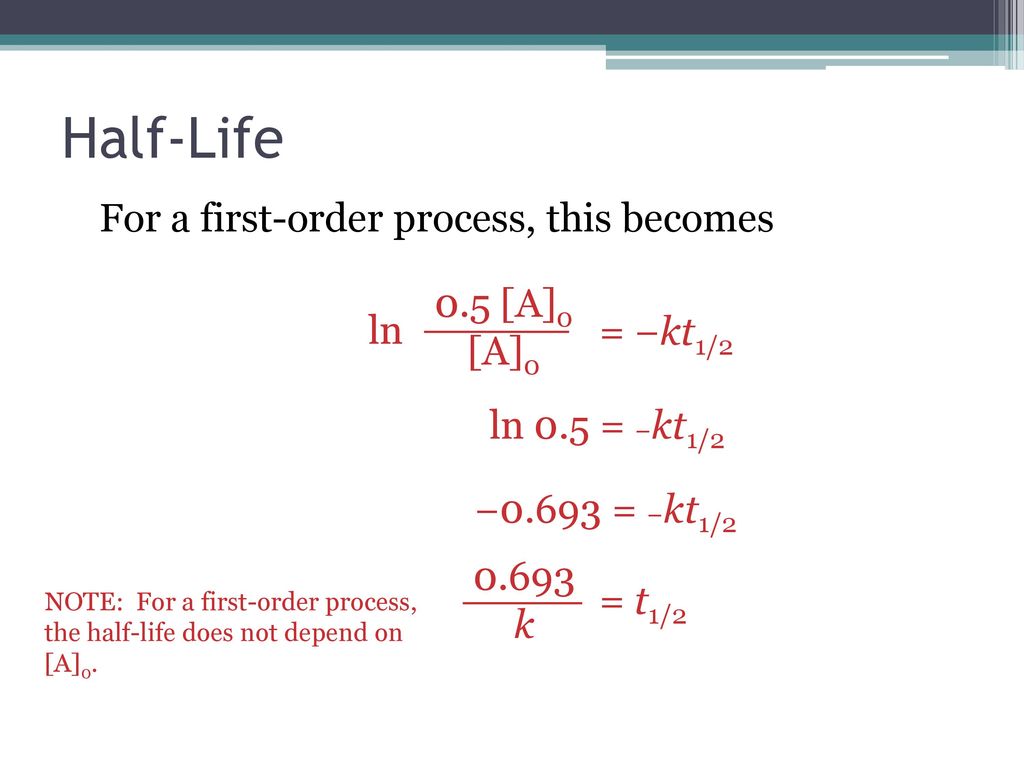



T1 2 Half Life Chemical Kinetics Ppt Download



Half Lives




For A Zero Order Reaction A Rarr B T 1 2 Is K Is Rate Constant




Concentration Time Graphs Ocr A Level Chemistry Teaching Resources



Link Springer Com




C7 Half Life Calculations N Noe Lamda T T1 2 Ln2 Lamda Hl Ib Chemistry Youtube




Ppt Rates Of Growth Decay Powerpoint Presentation Free Download Id
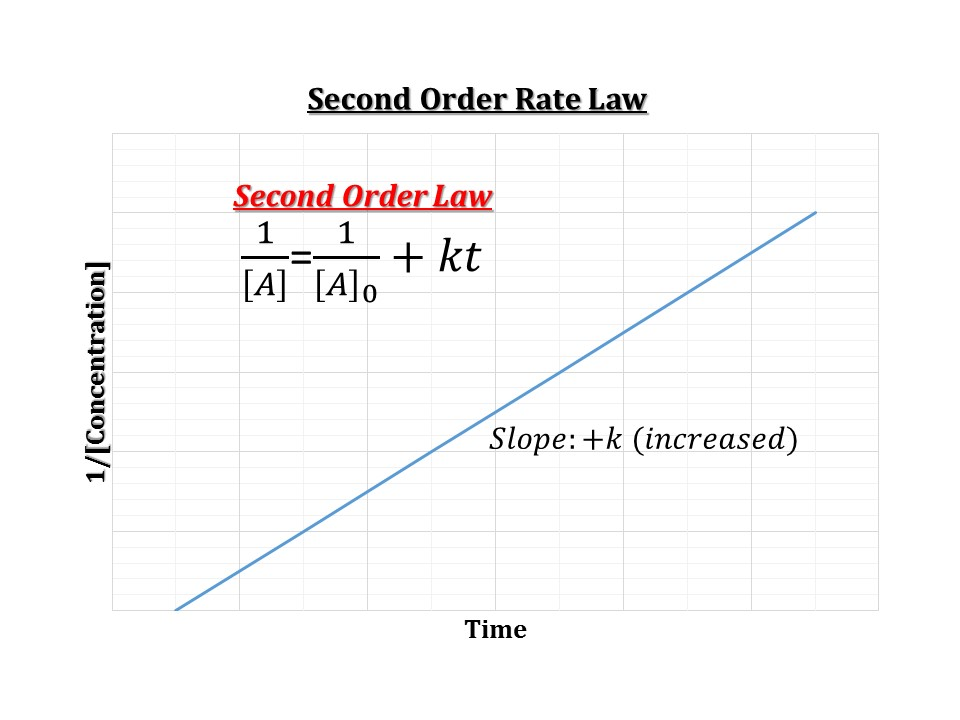



Help With Chem 2 Lab Please Thanks Half Life Chegg Com




Demi Vie




Solved For A First Order Reaction A P T1 2 Half Life Is 10 Days The Time Required For 1 Th 4 Conversion Of A In Days Is Ln 2 0 693 Ln 3 1 1



1



Half Life Deranged Physiology




What Is The Half Life Of 6c14 If Its Disintegration Constant Is 2 31 X 10 4 Yr 1 Chemical Kinetics



2
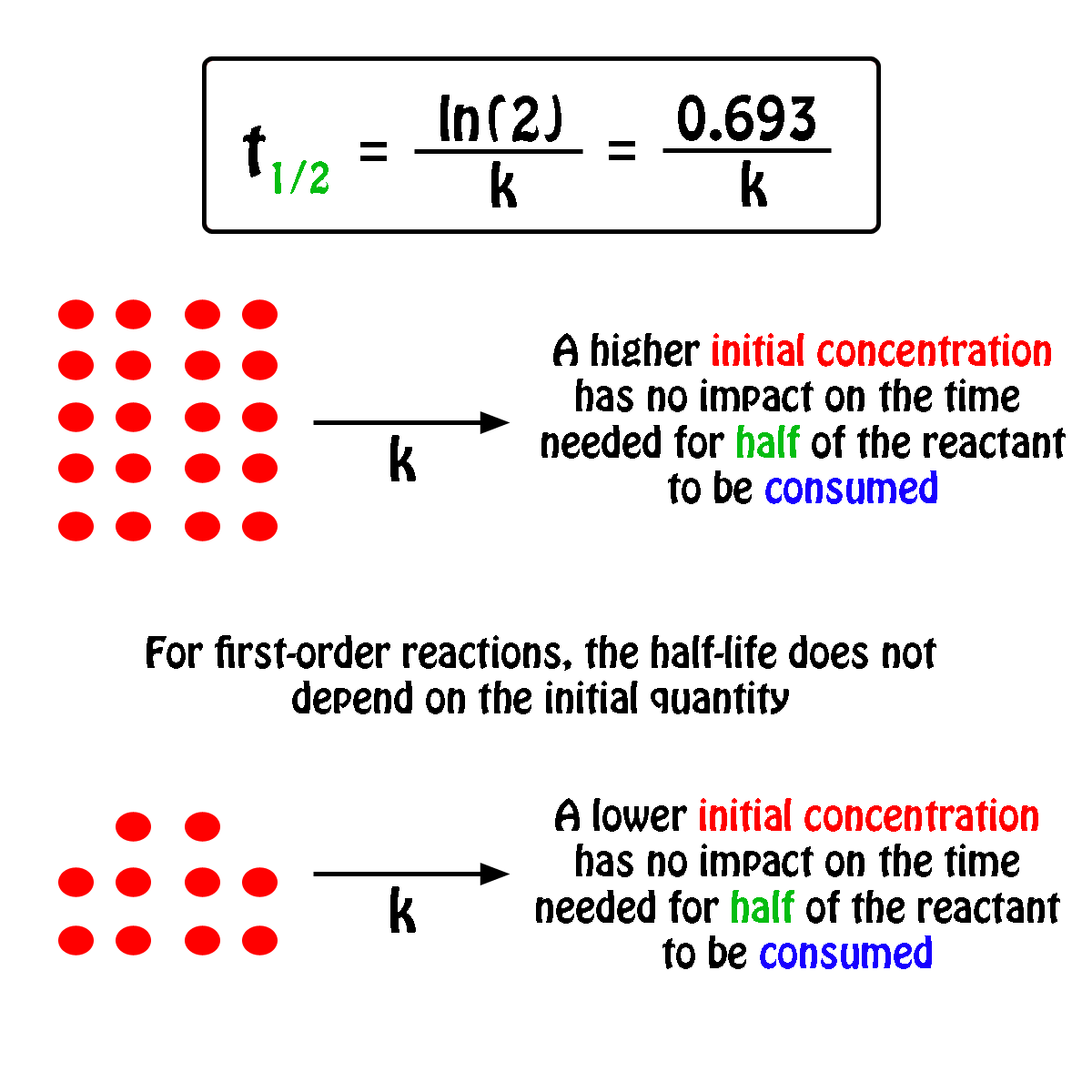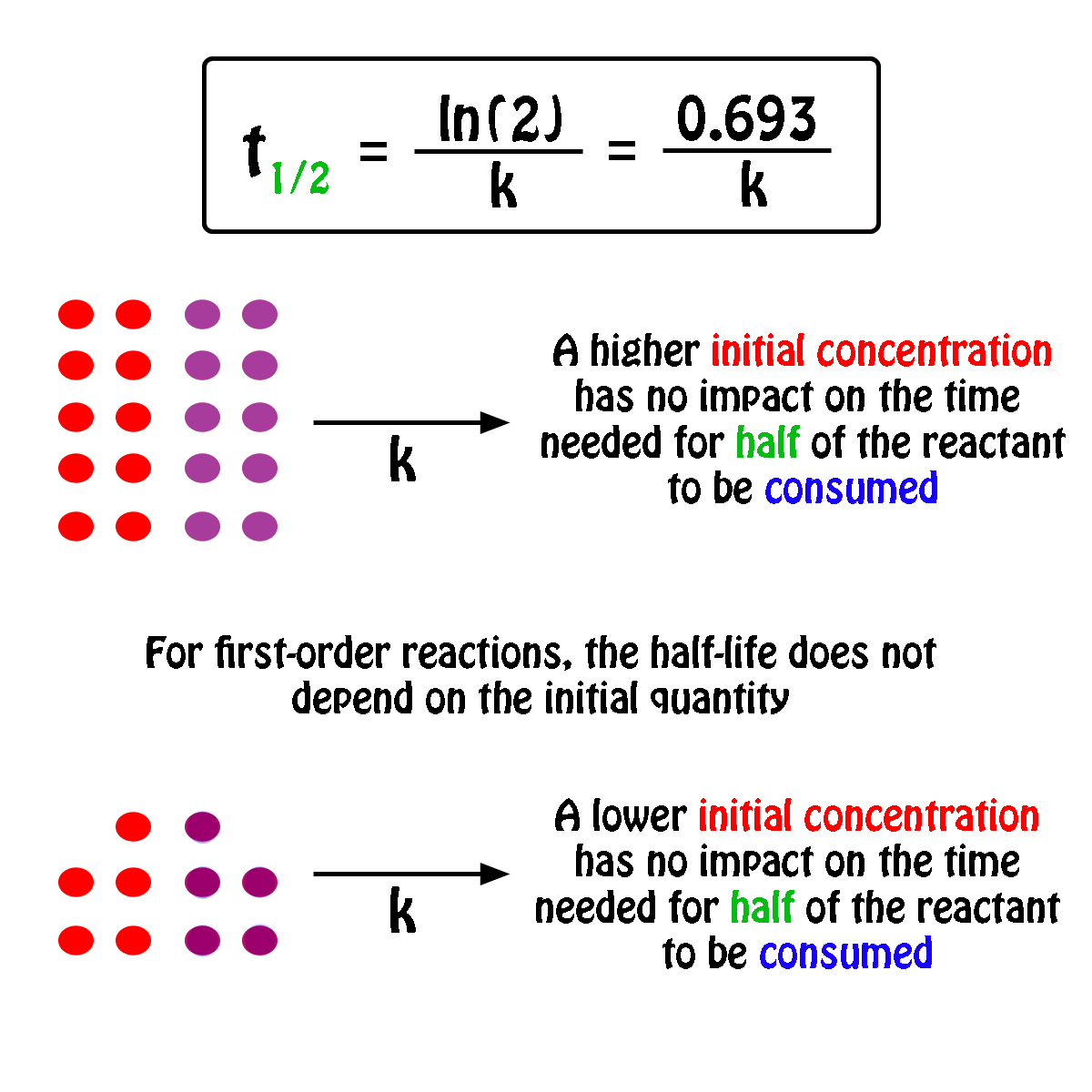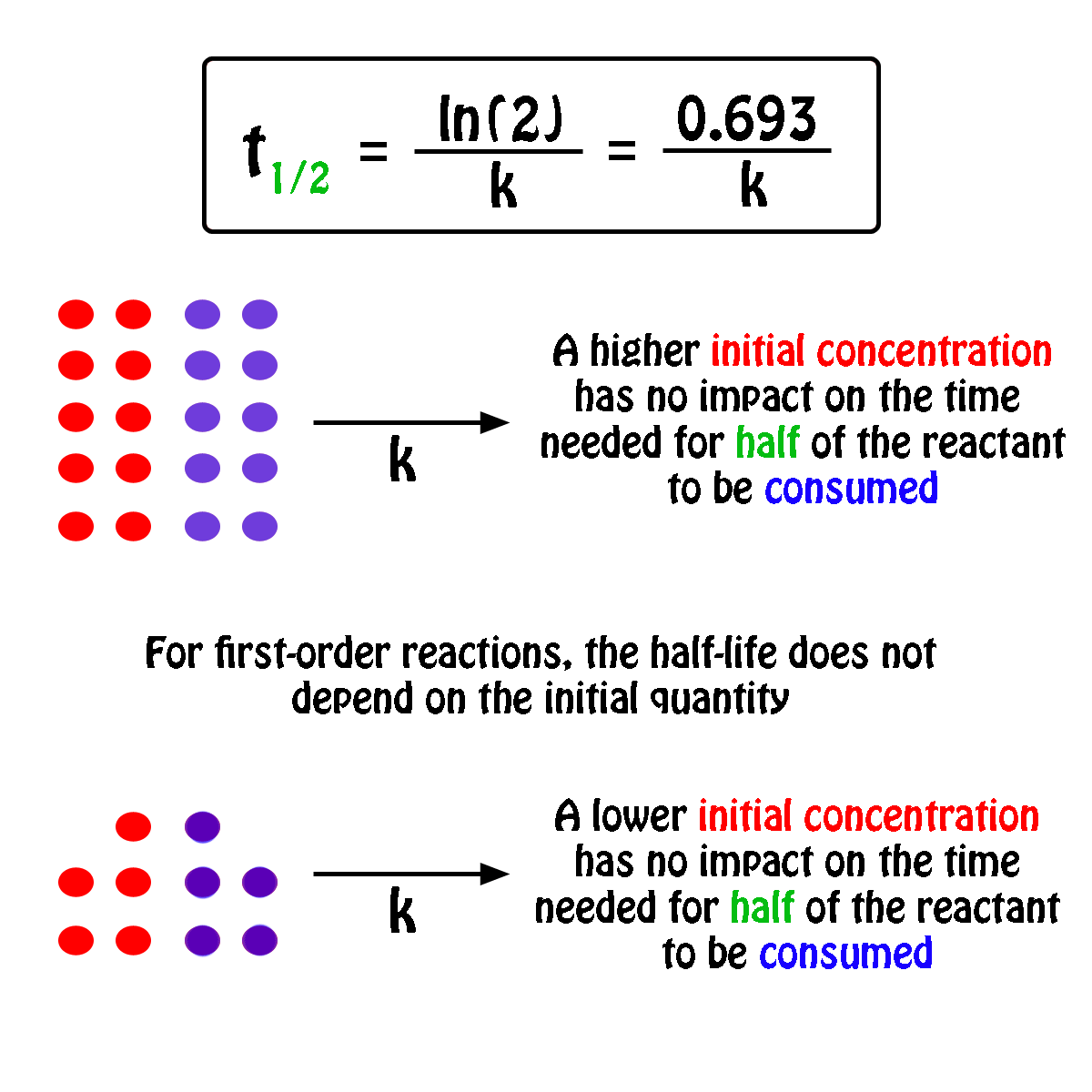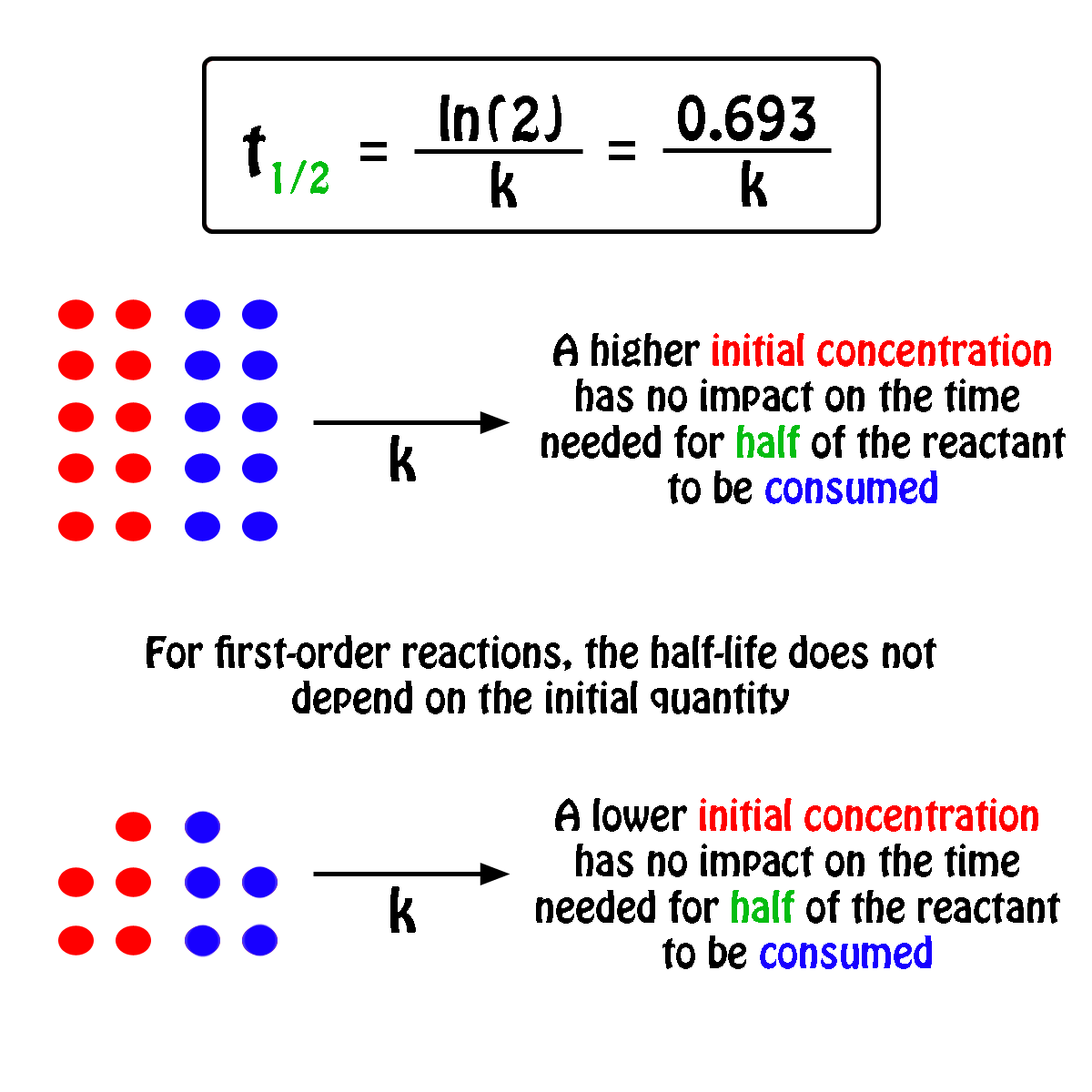



What Is The Half Life Of A First Order Reaction With A Rate Constant Of 7 80xx10 4 S 1 Socratic




Tga Curves Ta Instruments Bioz Ratings For Life Science Research



0 件のコメント:
コメントを投稿